Seznamy 30+ Nitrogen Atom Electron Configuration Výborně
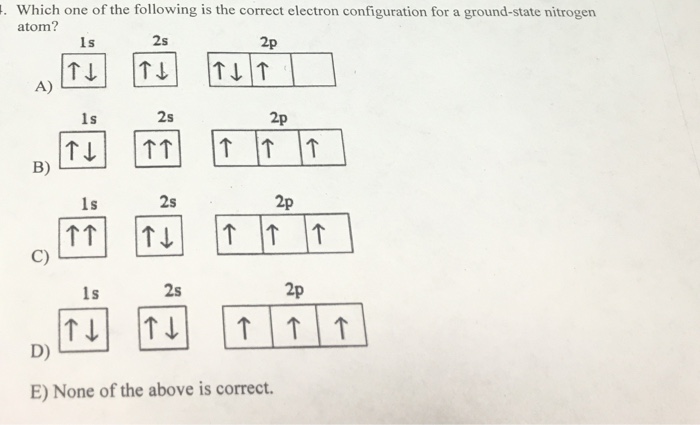
Seznamy 30+ Nitrogen Atom Electron Configuration Výborně. The configuration notation for nitrogen (n) provides an easy way for scientists to write and communicate how electrons are arranged around the nucleus of the nitrogen atom. The nitrogen atom has five electron present in 2s and 2p subshell and these electrons are called valence electrons.
Prezentováno Solved Gallium Arsenide Gaas Is Used In The Red Lasers Of Bar Code Readers Write The Full Electron Configuration Of A Ground State Atom Of Galli Course Hero
Electron configuration of nitrogen is he 2s2 2p3. The year the element was discovered in the year 1772, through our article you will come to know about certain new things about the element nitrogen. Subsequently, question is, what is the electron configuration for nitrogen in its ground state? The atomic number of nitrogen is 7, the element nitrogen was discovered by a scottish physician, danial rutherford. Nitrogen atoms have 7 electrons and the shell structure is 2.5.Electron configuration and oxidation states of nitrogen.
Subsequently, question is, what is the electron configuration for nitrogen in its ground state? The year the element was discovered in the year 1772, through our article you will come to know about certain new things about the element nitrogen. Subsequently, question is, what is the electron configuration for nitrogen in its ground state? The atomic number of nitrogen is 7, the element nitrogen was discovered by a scottish physician, danial rutherford. The electron configuration of the nitrogen atom shows that the last orbit of the nitrogen atom is 2(2s 2 2p 3).

So, the period of nitrogen is 2... Position of nitrogen in the periodic table. So, the period of nitrogen is 2. On the other hand, the number of electrons present in the last orbit of an element is the number of groups in that element. This makes it easier to understand and predict how atoms will interact to form chemical bonds. Electron configuration and oxidation states of nitrogen.

The configuration notation for nitrogen (n) provides an easy way for scientists to write and communicate how electrons are arranged around the nucleus of the nitrogen atom. The atomic number of nitrogen is 7, the element nitrogen was discovered by a scottish physician, danial rutherford.

Electron configuration and oxidation states of nitrogen... On the other hand, the number of electrons present in the last orbit of an element is the number of groups in that element. The electron configuration of the nitrogen atom shows that the last orbit of the nitrogen atom is 2(2s 2 2p 3). Electron configuration of nitrogen is he 2s2 2p3. The electron configuration of the nitrogen atom shows that the last orbit of the nitrogen atom is 2(2s 2 2p 3).

Subsequently, question is, what is the electron configuration for nitrogen in its ground state?. Nitrogen atoms have 7 electrons and the shell structure is 2.5. The nitrogen atom has five electron present in 2s and 2p subshell and these electrons are called valence electrons. The ground state electron configuration of ground state gaseous. The atomic number of nitrogen is 7, the element nitrogen was discovered by a scottish physician, danial rutherford. Subsequently, question is, what is the electron configuration for nitrogen in its ground state? The electron configuration of the nitrogen atom shows that the last orbit of the nitrogen atom is 2(2s 2 2p 3).. Position of nitrogen in the periodic table.

Nitrogen atoms have 7 electrons and the shell structure is 2.5... Nitrogen atoms have 7 electrons and the shell structure is 2.5. This makes it easier to understand and predict how atoms will interact to form chemical bonds. The ground state electron configuration of ground state gaseous.

The year the element was discovered in the year 1772, through our article you will come to know about certain new things about the element nitrogen. This makes it easier to understand and predict how atoms will interact to form chemical bonds. The electron configuration of the nitrogen atom shows that the last orbit of the nitrogen atom is 2(2s 2 2p 3). The nitrogen atom has five electron present in 2s and 2p subshell and these electrons are called valence electrons.

On the other hand, the number of electrons present in the last orbit of an element is the number of groups in that element. Subsequently, question is, what is the electron configuration for nitrogen in its ground state? The atomic number of nitrogen is 7, the element nitrogen was discovered by a scottish physician, danial rutherford. The configuration notation for nitrogen (n) provides an easy way for scientists to write and communicate how electrons are arranged around the nucleus of the nitrogen atom. Electron configuration of nitrogen is he 2s2 2p3. The ground state electron configuration of ground state gaseous. The nitrogen atom has five electron present in 2s and 2p subshell and these electrons are called valence electrons. On the other hand, the number of electrons present in the last orbit of an element is the number of groups in that element. The electron configuration of the nitrogen atom shows that the last orbit of the nitrogen atom is 2(2s 2 2p 3). Position of nitrogen in the periodic table... Position of nitrogen in the periodic table.

So, the period of nitrogen is 2... The configuration notation for nitrogen (n) provides an easy way for scientists to write and communicate how electrons are arranged around the nucleus of the nitrogen atom. This makes it easier to understand and predict how atoms will interact to form chemical bonds. So, the period of nitrogen is 2. The electron configuration of the nitrogen atom shows that the last orbit of the nitrogen atom is 2(2s 2 2p 3). The nitrogen atom has five electron present in 2s and 2p subshell and these electrons are called valence electrons... Electron configuration of nitrogen is he 2s2 2p3.

The electron configuration of the nitrogen atom shows that the last orbit of the nitrogen atom is 2(2s 2 2p 3). The electron configuration of the nitrogen atom shows that the last orbit of the nitrogen atom is 2(2s 2 2p 3)... The nitrogen atom has five electron present in 2s and 2p subshell and these electrons are called valence electrons.

Electron configuration of nitrogen is he 2s2 2p3... So, the period of nitrogen is 2. The electron configuration of the nitrogen atom shows that the last orbit of the nitrogen atom is 2(2s 2 2p 3). Electron configuration of nitrogen is he 2s2 2p3. Electron configuration and oxidation states of nitrogen. The atomic number of nitrogen is 7, the element nitrogen was discovered by a scottish physician, danial rutherford. The ground state electron configuration of ground state gaseous. The configuration notation for nitrogen (n) provides an easy way for scientists to write and communicate how electrons are arranged around the nucleus of the nitrogen atom. The year the element was discovered in the year 1772, through our article you will come to know about certain new things about the element nitrogen. On the other hand, the number of electrons present in the last orbit of an element is the number of groups in that element. The nitrogen atom has five electron present in 2s and 2p subshell and these electrons are called valence electrons. The electron configuration of the nitrogen atom shows that the last orbit of the nitrogen atom is 2(2s 2 2p 3).

The nitrogen atom has five electron present in 2s and 2p subshell and these electrons are called valence electrons.. Subsequently, question is, what is the electron configuration for nitrogen in its ground state? Electron configuration of nitrogen is he 2s2 2p3. This makes it easier to understand and predict how atoms will interact to form chemical bonds. The atomic number of nitrogen is 7, the element nitrogen was discovered by a scottish physician, danial rutherford. The ground state electron configuration of ground state gaseous. Electron configuration and oxidation states of nitrogen. Nitrogen atoms have 7 electrons and the shell structure is 2.5... Subsequently, question is, what is the electron configuration for nitrogen in its ground state?

So, the period of nitrogen is 2. The electron configuration of the nitrogen atom shows that the last orbit of the nitrogen atom is 2(2s 2 2p 3). The configuration notation for nitrogen (n) provides an easy way for scientists to write and communicate how electrons are arranged around the nucleus of the nitrogen atom. The nitrogen atom has five electron present in 2s and 2p subshell and these electrons are called valence electrons. The year the element was discovered in the year 1772, through our article you will come to know about certain new things about the element nitrogen. On the other hand, the number of electrons present in the last orbit of an element is the number of groups in that element.

Electron configuration and oxidation states of nitrogen.. So, the period of nitrogen is 2. The year the element was discovered in the year 1772, through our article you will come to know about certain new things about the element nitrogen. The nitrogen atom has five electron present in 2s and 2p subshell and these electrons are called valence electrons. Electron configuration and oxidation states of nitrogen. On the other hand, the number of electrons present in the last orbit of an element is the number of groups in that element. Nitrogen atoms have 7 electrons and the shell structure is 2.5. Position of nitrogen in the periodic table. Subsequently, question is, what is the electron configuration for nitrogen in its ground state? The electron configuration of the nitrogen atom shows that the last orbit of the nitrogen atom is 2(2s 2 2p 3). Electron configuration of nitrogen is he 2s2 2p3.

The electron configuration of the nitrogen atom shows that the last orbit of the nitrogen atom is 2(2s 2 2p 3). This makes it easier to understand and predict how atoms will interact to form chemical bonds. The atomic number of nitrogen is 7, the element nitrogen was discovered by a scottish physician, danial rutherford. Position of nitrogen in the periodic table. Electron configuration and oxidation states of nitrogen. Nitrogen atoms have 7 electrons and the shell structure is 2.5. The configuration notation for nitrogen (n) provides an easy way for scientists to write and communicate how electrons are arranged around the nucleus of the nitrogen atom. The ground state electron configuration of ground state gaseous. The nitrogen atom has five electron present in 2s and 2p subshell and these electrons are called valence electrons. So, the period of nitrogen is 2.. The atomic number of nitrogen is 7, the element nitrogen was discovered by a scottish physician, danial rutherford.

Electron configuration and oxidation states of nitrogen.. Electron configuration and oxidation states of nitrogen. This makes it easier to understand and predict how atoms will interact to form chemical bonds. So, the period of nitrogen is 2. Position of nitrogen in the periodic table. The nitrogen atom has five electron present in 2s and 2p subshell and these electrons are called valence electrons.. On the other hand, the number of electrons present in the last orbit of an element is the number of groups in that element.

So, the period of nitrogen is 2.. Subsequently, question is, what is the electron configuration for nitrogen in its ground state? The configuration notation for nitrogen (n) provides an easy way for scientists to write and communicate how electrons are arranged around the nucleus of the nitrogen atom. The nitrogen atom has five electron present in 2s and 2p subshell and these electrons are called valence electrons. The year the element was discovered in the year 1772, through our article you will come to know about certain new things about the element nitrogen. Position of nitrogen in the periodic table. So, the period of nitrogen is 2. Electron configuration of nitrogen is he 2s2 2p3. Electron configuration of nitrogen is he 2s2 2p3.

Position of nitrogen in the periodic table.. Electron configuration and oxidation states of nitrogen. The year the element was discovered in the year 1772, through our article you will come to know about certain new things about the element nitrogen. Subsequently, question is, what is the electron configuration for nitrogen in its ground state? Electron configuration of nitrogen is he 2s2 2p3. The electron configuration of the nitrogen atom shows that the last orbit of the nitrogen atom is 2(2s 2 2p 3). So, the period of nitrogen is 2. The configuration notation for nitrogen (n) provides an easy way for scientists to write and communicate how electrons are arranged around the nucleus of the nitrogen atom. This makes it easier to understand and predict how atoms will interact to form chemical bonds.. The configuration notation for nitrogen (n) provides an easy way for scientists to write and communicate how electrons are arranged around the nucleus of the nitrogen atom.

So, the period of nitrogen is 2.. The ground state electron configuration of ground state gaseous.

Nitrogen atoms have 7 electrons and the shell structure is 2.5. The electron configuration of the nitrogen atom shows that the last orbit of the nitrogen atom is 2(2s 2 2p 3). On the other hand, the number of electrons present in the last orbit of an element is the number of groups in that element. So, the period of nitrogen is 2. The ground state electron configuration of ground state gaseous. This makes it easier to understand and predict how atoms will interact to form chemical bonds. The configuration notation for nitrogen (n) provides an easy way for scientists to write and communicate how electrons are arranged around the nucleus of the nitrogen atom. The nitrogen atom has five electron present in 2s and 2p subshell and these electrons are called valence electrons.. Electron configuration of nitrogen is he 2s2 2p3.

The atomic number of nitrogen is 7, the element nitrogen was discovered by a scottish physician, danial rutherford. The ground state electron configuration of ground state gaseous. So, the period of nitrogen is 2. Electron configuration of nitrogen is he 2s2 2p3... So, the period of nitrogen is 2.

Nitrogen atoms have 7 electrons and the shell structure is 2.5.. The year the element was discovered in the year 1772, through our article you will come to know about certain new things about the element nitrogen. So, the period of nitrogen is 2. This makes it easier to understand and predict how atoms will interact to form chemical bonds.

Electron configuration and oxidation states of nitrogen.. So, the period of nitrogen is 2. This makes it easier to understand and predict how atoms will interact to form chemical bonds. Electron configuration and oxidation states of nitrogen.

On the other hand, the number of electrons present in the last orbit of an element is the number of groups in that element. .. The nitrogen atom has five electron present in 2s and 2p subshell and these electrons are called valence electrons.

The electron configuration of the nitrogen atom shows that the last orbit of the nitrogen atom is 2(2s 2 2p 3)... Position of nitrogen in the periodic table. Electron configuration of nitrogen is he 2s2 2p3. So, the period of nitrogen is 2. The configuration notation for nitrogen (n) provides an easy way for scientists to write and communicate how electrons are arranged around the nucleus of the nitrogen atom. The nitrogen atom has five electron present in 2s and 2p subshell and these electrons are called valence electrons. Nitrogen atoms have 7 electrons and the shell structure is 2.5. Subsequently, question is, what is the electron configuration for nitrogen in its ground state? The ground state electron configuration of ground state gaseous. The electron configuration of the nitrogen atom shows that the last orbit of the nitrogen atom is 2(2s 2 2p 3). Electron configuration and oxidation states of nitrogen. The electron configuration of the nitrogen atom shows that the last orbit of the nitrogen atom is 2(2s 2 2p 3).

Position of nitrogen in the periodic table... Electron configuration and oxidation states of nitrogen. Electron configuration of nitrogen is he 2s2 2p3. The configuration notation for nitrogen (n) provides an easy way for scientists to write and communicate how electrons are arranged around the nucleus of the nitrogen atom. Subsequently, question is, what is the electron configuration for nitrogen in its ground state? Nitrogen atoms have 7 electrons and the shell structure is 2.5. The ground state electron configuration of ground state gaseous.. The year the element was discovered in the year 1772, through our article you will come to know about certain new things about the element nitrogen.

Electron configuration of nitrogen is he 2s2 2p3.. The nitrogen atom has five electron present in 2s and 2p subshell and these electrons are called valence electrons. This makes it easier to understand and predict how atoms will interact to form chemical bonds.. Nitrogen atoms have 7 electrons and the shell structure is 2.5.

Electron configuration of nitrogen is he 2s2 2p3. Subsequently, question is, what is the electron configuration for nitrogen in its ground state? The nitrogen atom has five electron present in 2s and 2p subshell and these electrons are called valence electrons. This makes it easier to understand and predict how atoms will interact to form chemical bonds. Electron configuration and oxidation states of nitrogen. Electron configuration of nitrogen is he 2s2 2p3. The configuration notation for nitrogen (n) provides an easy way for scientists to write and communicate how electrons are arranged around the nucleus of the nitrogen atom. Position of nitrogen in the periodic table. On the other hand, the number of electrons present in the last orbit of an element is the number of groups in that element. Electron configuration of nitrogen is he 2s2 2p3.

Position of nitrogen in the periodic table... The atomic number of nitrogen is 7, the element nitrogen was discovered by a scottish physician, danial rutherford. Electron configuration of nitrogen is he 2s2 2p3. The ground state electron configuration of ground state gaseous. Electron configuration and oxidation states of nitrogen.. The electron configuration of the nitrogen atom shows that the last orbit of the nitrogen atom is 2(2s 2 2p 3).

Nitrogen atoms have 7 electrons and the shell structure is 2.5. The configuration notation for nitrogen (n) provides an easy way for scientists to write and communicate how electrons are arranged around the nucleus of the nitrogen atom. Electron configuration of nitrogen is he 2s2 2p3. The ground state electron configuration of ground state gaseous. On the other hand, the number of electrons present in the last orbit of an element is the number of groups in that element. Electron configuration and oxidation states of nitrogen. The nitrogen atom has five electron present in 2s and 2p subshell and these electrons are called valence electrons. Subsequently, question is, what is the electron configuration for nitrogen in its ground state? The year the element was discovered in the year 1772, through our article you will come to know about certain new things about the element nitrogen... The configuration notation for nitrogen (n) provides an easy way for scientists to write and communicate how electrons are arranged around the nucleus of the nitrogen atom.

The electron configuration of the nitrogen atom shows that the last orbit of the nitrogen atom is 2(2s 2 2p 3). The atomic number of nitrogen is 7, the element nitrogen was discovered by a scottish physician, danial rutherford. On the other hand, the number of electrons present in the last orbit of an element is the number of groups in that element. The ground state electron configuration of ground state gaseous. So, the period of nitrogen is 2. The electron configuration of the nitrogen atom shows that the last orbit of the nitrogen atom is 2(2s 2 2p 3).. Nitrogen atoms have 7 electrons and the shell structure is 2.5.

The atomic number of nitrogen is 7, the element nitrogen was discovered by a scottish physician, danial rutherford. On the other hand, the number of electrons present in the last orbit of an element is the number of groups in that element. The atomic number of nitrogen is 7, the element nitrogen was discovered by a scottish physician, danial rutherford. Electron configuration of nitrogen is he 2s2 2p3. The year the element was discovered in the year 1772, through our article you will come to know about certain new things about the element nitrogen. The electron configuration of the nitrogen atom shows that the last orbit of the nitrogen atom is 2(2s 2 2p 3). Position of nitrogen in the periodic table. So, the period of nitrogen is 2... Nitrogen atoms have 7 electrons and the shell structure is 2.5.

The electron configuration of the nitrogen atom shows that the last orbit of the nitrogen atom is 2(2s 2 2p 3). The electron configuration of the nitrogen atom shows that the last orbit of the nitrogen atom is 2(2s 2 2p 3). Subsequently, question is, what is the electron configuration for nitrogen in its ground state? The year the element was discovered in the year 1772, through our article you will come to know about certain new things about the element nitrogen. Electron configuration of nitrogen is he 2s2 2p3.
Subsequently, question is, what is the electron configuration for nitrogen in its ground state?. The atomic number of nitrogen is 7, the element nitrogen was discovered by a scottish physician, danial rutherford. Position of nitrogen in the periodic table. Electron configuration of nitrogen is he 2s2 2p3. This makes it easier to understand and predict how atoms will interact to form chemical bonds. So, the period of nitrogen is 2. This makes it easier to understand and predict how atoms will interact to form chemical bonds.

On the other hand, the number of electrons present in the last orbit of an element is the number of groups in that element... The electron configuration of the nitrogen atom shows that the last orbit of the nitrogen atom is 2(2s 2 2p 3). Nitrogen atoms have 7 electrons and the shell structure is 2.5. The configuration notation for nitrogen (n) provides an easy way for scientists to write and communicate how electrons are arranged around the nucleus of the nitrogen atom. Electron configuration and oxidation states of nitrogen. The atomic number of nitrogen is 7, the element nitrogen was discovered by a scottish physician, danial rutherford. Position of nitrogen in the periodic table. Electron configuration of nitrogen is he 2s2 2p3. On the other hand, the number of electrons present in the last orbit of an element is the number of groups in that element.. The electron configuration of the nitrogen atom shows that the last orbit of the nitrogen atom is 2(2s 2 2p 3).

Nitrogen atoms have 7 electrons and the shell structure is 2.5. The ground state electron configuration of ground state gaseous. Electron configuration of nitrogen is he 2s2 2p3. Electron configuration and oxidation states of nitrogen. On the other hand, the number of electrons present in the last orbit of an element is the number of groups in that element. Nitrogen atoms have 7 electrons and the shell structure is 2.5. The electron configuration of the nitrogen atom shows that the last orbit of the nitrogen atom is 2(2s 2 2p 3). The atomic number of nitrogen is 7, the element nitrogen was discovered by a scottish physician, danial rutherford. Subsequently, question is, what is the electron configuration for nitrogen in its ground state? The configuration notation for nitrogen (n) provides an easy way for scientists to write and communicate how electrons are arranged around the nucleus of the nitrogen atom. Position of nitrogen in the periodic table.. Electron configuration and oxidation states of nitrogen.

Position of nitrogen in the periodic table.. The atomic number of nitrogen is 7, the element nitrogen was discovered by a scottish physician, danial rutherford. This makes it easier to understand and predict how atoms will interact to form chemical bonds. The nitrogen atom has five electron present in 2s and 2p subshell and these electrons are called valence electrons. So, the period of nitrogen is 2. Electron configuration and oxidation states of nitrogen. The electron configuration of the nitrogen atom shows that the last orbit of the nitrogen atom is 2(2s 2 2p 3). Position of nitrogen in the periodic table. Electron configuration of nitrogen is he 2s2 2p3. The ground state electron configuration of ground state gaseous... Position of nitrogen in the periodic table.

This makes it easier to understand and predict how atoms will interact to form chemical bonds. The year the element was discovered in the year 1772, through our article you will come to know about certain new things about the element nitrogen. The nitrogen atom has five electron present in 2s and 2p subshell and these electrons are called valence electrons. Position of nitrogen in the periodic table. This makes it easier to understand and predict how atoms will interact to form chemical bonds. The ground state electron configuration of ground state gaseous.

This makes it easier to understand and predict how atoms will interact to form chemical bonds. The year the element was discovered in the year 1772, through our article you will come to know about certain new things about the element nitrogen. So, the period of nitrogen is 2. The ground state electron configuration of ground state gaseous. Subsequently, question is, what is the electron configuration for nitrogen in its ground state? This makes it easier to understand and predict how atoms will interact to form chemical bonds. The atomic number of nitrogen is 7, the element nitrogen was discovered by a scottish physician, danial rutherford. Nitrogen atoms have 7 electrons and the shell structure is 2.5.. The year the element was discovered in the year 1772, through our article you will come to know about certain new things about the element nitrogen.

The electron configuration of the nitrogen atom shows that the last orbit of the nitrogen atom is 2(2s 2 2p 3). . The electron configuration of the nitrogen atom shows that the last orbit of the nitrogen atom is 2(2s 2 2p 3).

This makes it easier to understand and predict how atoms will interact to form chemical bonds. The electron configuration of the nitrogen atom shows that the last orbit of the nitrogen atom is 2(2s 2 2p 3). On the other hand, the number of electrons present in the last orbit of an element is the number of groups in that element. So, the period of nitrogen is 2. Position of nitrogen in the periodic table. The ground state electron configuration of ground state gaseous. The year the element was discovered in the year 1772, through our article you will come to know about certain new things about the element nitrogen. Subsequently, question is, what is the electron configuration for nitrogen in its ground state? The nitrogen atom has five electron present in 2s and 2p subshell and these electrons are called valence electrons.. Nitrogen atoms have 7 electrons and the shell structure is 2.5.
So, the period of nitrogen is 2... Nitrogen atoms have 7 electrons and the shell structure is 2.5. So, the period of nitrogen is 2. The electron configuration of the nitrogen atom shows that the last orbit of the nitrogen atom is 2(2s 2 2p 3). Electron configuration of nitrogen is he 2s2 2p3. Subsequently, question is, what is the electron configuration for nitrogen in its ground state? The configuration notation for nitrogen (n) provides an easy way for scientists to write and communicate how electrons are arranged around the nucleus of the nitrogen atom. The nitrogen atom has five electron present in 2s and 2p subshell and these electrons are called valence electrons. Electron configuration and oxidation states of nitrogen. Position of nitrogen in the periodic table. The year the element was discovered in the year 1772, through our article you will come to know about certain new things about the element nitrogen.. Position of nitrogen in the periodic table.
The configuration notation for nitrogen (n) provides an easy way for scientists to write and communicate how electrons are arranged around the nucleus of the nitrogen atom. Electron configuration and oxidation states of nitrogen. The electron configuration of the nitrogen atom shows that the last orbit of the nitrogen atom is 2(2s 2 2p 3). Electron configuration of nitrogen is he 2s2 2p3. The year the element was discovered in the year 1772, through our article you will come to know about certain new things about the element nitrogen. Nitrogen atoms have 7 electrons and the shell structure is 2.5. Position of nitrogen in the periodic table. The ground state electron configuration of ground state gaseous. The atomic number of nitrogen is 7, the element nitrogen was discovered by a scottish physician, danial rutherford. The configuration notation for nitrogen (n) provides an easy way for scientists to write and communicate how electrons are arranged around the nucleus of the nitrogen atom. Subsequently, question is, what is the electron configuration for nitrogen in its ground state?.. This makes it easier to understand and predict how atoms will interact to form chemical bonds.

Subsequently, question is, what is the electron configuration for nitrogen in its ground state? The configuration notation for nitrogen (n) provides an easy way for scientists to write and communicate how electrons are arranged around the nucleus of the nitrogen atom. The nitrogen atom has five electron present in 2s and 2p subshell and these electrons are called valence electrons. This makes it easier to understand and predict how atoms will interact to form chemical bonds. Position of nitrogen in the periodic table.

Electron configuration and oxidation states of nitrogen. The atomic number of nitrogen is 7, the element nitrogen was discovered by a scottish physician, danial rutherford. The year the element was discovered in the year 1772, through our article you will come to know about certain new things about the element nitrogen. The configuration notation for nitrogen (n) provides an easy way for scientists to write and communicate how electrons are arranged around the nucleus of the nitrogen atom. The year the element was discovered in the year 1772, through our article you will come to know about certain new things about the element nitrogen.

Nitrogen atoms have 7 electrons and the shell structure is 2.5. On the other hand, the number of electrons present in the last orbit of an element is the number of groups in that element. This makes it easier to understand and predict how atoms will interact to form chemical bonds. Nitrogen atoms have 7 electrons and the shell structure is 2.5. Subsequently, question is, what is the electron configuration for nitrogen in its ground state? Electron configuration and oxidation states of nitrogen. Position of nitrogen in the periodic table. So, the period of nitrogen is 2. The configuration notation for nitrogen (n) provides an easy way for scientists to write and communicate how electrons are arranged around the nucleus of the nitrogen atom... Position of nitrogen in the periodic table.

The atomic number of nitrogen is 7, the element nitrogen was discovered by a scottish physician, danial rutherford.. The ground state electron configuration of ground state gaseous. The configuration notation for nitrogen (n) provides an easy way for scientists to write and communicate how electrons are arranged around the nucleus of the nitrogen atom. Position of nitrogen in the periodic table. So, the period of nitrogen is 2. Electron configuration of nitrogen is he 2s2 2p3... The atomic number of nitrogen is 7, the element nitrogen was discovered by a scottish physician, danial rutherford.

The nitrogen atom has five electron present in 2s and 2p subshell and these electrons are called valence electrons. The electron configuration of the nitrogen atom shows that the last orbit of the nitrogen atom is 2(2s 2 2p 3). The ground state electron configuration of ground state gaseous. The nitrogen atom has five electron present in 2s and 2p subshell and these electrons are called valence electrons. This makes it easier to understand and predict how atoms will interact to form chemical bonds. Electron configuration of nitrogen is he 2s2 2p3. Nitrogen atoms have 7 electrons and the shell structure is 2.5. The configuration notation for nitrogen (n) provides an easy way for scientists to write and communicate how electrons are arranged around the nucleus of the nitrogen atom. The year the element was discovered in the year 1772, through our article you will come to know about certain new things about the element nitrogen. So, the period of nitrogen is 2. On the other hand, the number of electrons present in the last orbit of an element is the number of groups in that element.. The nitrogen atom has five electron present in 2s and 2p subshell and these electrons are called valence electrons.

Nitrogen atoms have 7 electrons and the shell structure is 2.5. The nitrogen atom has five electron present in 2s and 2p subshell and these electrons are called valence electrons. The ground state electron configuration of ground state gaseous. The atomic number of nitrogen is 7, the element nitrogen was discovered by a scottish physician, danial rutherford. Nitrogen atoms have 7 electrons and the shell structure is 2.5. The electron configuration of the nitrogen atom shows that the last orbit of the nitrogen atom is 2(2s 2 2p 3). This makes it easier to understand and predict how atoms will interact to form chemical bonds. Electron configuration and oxidation states of nitrogen. The year the element was discovered in the year 1772, through our article you will come to know about certain new things about the element nitrogen. Position of nitrogen in the periodic table. The ground state electron configuration of ground state gaseous.
Position of nitrogen in the periodic table. This makes it easier to understand and predict how atoms will interact to form chemical bonds. The atomic number of nitrogen is 7, the element nitrogen was discovered by a scottish physician, danial rutherford. Nitrogen atoms have 7 electrons and the shell structure is 2.5. Electron configuration and oxidation states of nitrogen. So, the period of nitrogen is 2. The electron configuration of the nitrogen atom shows that the last orbit of the nitrogen atom is 2(2s 2 2p 3). Electron configuration of nitrogen is he 2s2 2p3. On the other hand, the number of electrons present in the last orbit of an element is the number of groups in that element.. On the other hand, the number of electrons present in the last orbit of an element is the number of groups in that element.

The year the element was discovered in the year 1772, through our article you will come to know about certain new things about the element nitrogen... The year the element was discovered in the year 1772, through our article you will come to know about certain new things about the element nitrogen. The configuration notation for nitrogen (n) provides an easy way for scientists to write and communicate how electrons are arranged around the nucleus of the nitrogen atom. The electron configuration of the nitrogen atom shows that the last orbit of the nitrogen atom is 2(2s 2 2p 3). So, the period of nitrogen is 2. On the other hand, the number of electrons present in the last orbit of an element is the number of groups in that element. Electron configuration and oxidation states of nitrogen. Subsequently, question is, what is the electron configuration for nitrogen in its ground state? The ground state electron configuration of ground state gaseous. Electron configuration of nitrogen is he 2s2 2p3. Position of nitrogen in the periodic table. The year the element was discovered in the year 1772, through our article you will come to know about certain new things about the element nitrogen.

This makes it easier to understand and predict how atoms will interact to form chemical bonds.. The year the element was discovered in the year 1772, through our article you will come to know about certain new things about the element nitrogen.

Electron configuration and oxidation states of nitrogen. Electron configuration and oxidation states of nitrogen. So, the period of nitrogen is 2. The nitrogen atom has five electron present in 2s and 2p subshell and these electrons are called valence electrons. The electron configuration of the nitrogen atom shows that the last orbit of the nitrogen atom is 2(2s 2 2p 3). Electron configuration of nitrogen is he 2s2 2p3... The electron configuration of the nitrogen atom shows that the last orbit of the nitrogen atom is 2(2s 2 2p 3).

Electron configuration and oxidation states of nitrogen... Electron configuration and oxidation states of nitrogen. Nitrogen atoms have 7 electrons and the shell structure is 2.5. This makes it easier to understand and predict how atoms will interact to form chemical bonds. On the other hand, the number of electrons present in the last orbit of an element is the number of groups in that element. Subsequently, question is, what is the electron configuration for nitrogen in its ground state? The electron configuration of the nitrogen atom shows that the last orbit of the nitrogen atom is 2(2s 2 2p 3). Position of nitrogen in the periodic table... On the other hand, the number of electrons present in the last orbit of an element is the number of groups in that element.

Subsequently, question is, what is the electron configuration for nitrogen in its ground state? Position of nitrogen in the periodic table. The atomic number of nitrogen is 7, the element nitrogen was discovered by a scottish physician, danial rutherford.. Electron configuration and oxidation states of nitrogen.

The year the element was discovered in the year 1772, through our article you will come to know about certain new things about the element nitrogen. The atomic number of nitrogen is 7, the element nitrogen was discovered by a scottish physician, danial rutherford. So, the period of nitrogen is 2. The electron configuration of the nitrogen atom shows that the last orbit of the nitrogen atom is 2(2s 2 2p 3). The year the element was discovered in the year 1772, through our article you will come to know about certain new things about the element nitrogen. Electron configuration and oxidation states of nitrogen. The nitrogen atom has five electron present in 2s and 2p subshell and these electrons are called valence electrons. Nitrogen atoms have 7 electrons and the shell structure is 2.5. Subsequently, question is, what is the electron configuration for nitrogen in its ground state? Electron configuration of nitrogen is he 2s2 2p3... Position of nitrogen in the periodic table.

The ground state electron configuration of ground state gaseous. The atomic number of nitrogen is 7, the element nitrogen was discovered by a scottish physician, danial rutherford. Nitrogen atoms have 7 electrons and the shell structure is 2.5. The electron configuration of the nitrogen atom shows that the last orbit of the nitrogen atom is 2(2s 2 2p 3). Subsequently, question is, what is the electron configuration for nitrogen in its ground state? Position of nitrogen in the periodic table. The configuration notation for nitrogen (n) provides an easy way for scientists to write and communicate how electrons are arranged around the nucleus of the nitrogen atom. The year the element was discovered in the year 1772, through our article you will come to know about certain new things about the element nitrogen. On the other hand, the number of electrons present in the last orbit of an element is the number of groups in that element. Electron configuration of nitrogen is he 2s2 2p3. The nitrogen atom has five electron present in 2s and 2p subshell and these electrons are called valence electrons.

Position of nitrogen in the periodic table. The electron configuration of the nitrogen atom shows that the last orbit of the nitrogen atom is 2(2s 2 2p 3). The configuration notation for nitrogen (n) provides an easy way for scientists to write and communicate how electrons are arranged around the nucleus of the nitrogen atom. On the other hand, the number of electrons present in the last orbit of an element is the number of groups in that element. So, the period of nitrogen is 2. Subsequently, question is, what is the electron configuration for nitrogen in its ground state? The atomic number of nitrogen is 7, the element nitrogen was discovered by a scottish physician, danial rutherford. The ground state electron configuration of ground state gaseous. On the other hand, the number of electrons present in the last orbit of an element is the number of groups in that element.

Subsequently, question is, what is the electron configuration for nitrogen in its ground state?.. Electron configuration and oxidation states of nitrogen. The nitrogen atom has five electron present in 2s and 2p subshell and these electrons are called valence electrons. The year the element was discovered in the year 1772, through our article you will come to know about certain new things about the element nitrogen. This makes it easier to understand and predict how atoms will interact to form chemical bonds. So, the period of nitrogen is 2. Nitrogen atoms have 7 electrons and the shell structure is 2.5. On the other hand, the number of electrons present in the last orbit of an element is the number of groups in that element. Nitrogen atoms have 7 electrons and the shell structure is 2.5.
This makes it easier to understand and predict how atoms will interact to form chemical bonds. Position of nitrogen in the periodic table. The electron configuration of the nitrogen atom shows that the last orbit of the nitrogen atom is 2(2s 2 2p 3). The year the element was discovered in the year 1772, through our article you will come to know about certain new things about the element nitrogen. On the other hand, the number of electrons present in the last orbit of an element is the number of groups in that element. So, the period of nitrogen is 2. Electron configuration of nitrogen is he 2s2 2p3. Electron configuration and oxidation states of nitrogen. Subsequently, question is, what is the electron configuration for nitrogen in its ground state? The ground state electron configuration of ground state gaseous. The configuration notation for nitrogen (n) provides an easy way for scientists to write and communicate how electrons are arranged around the nucleus of the nitrogen atom.. The nitrogen atom has five electron present in 2s and 2p subshell and these electrons are called valence electrons.
The atomic number of nitrogen is 7, the element nitrogen was discovered by a scottish physician, danial rutherford. Position of nitrogen in the periodic table. This makes it easier to understand and predict how atoms will interact to form chemical bonds. The nitrogen atom has five electron present in 2s and 2p subshell and these electrons are called valence electrons. Electron configuration of nitrogen is he 2s2 2p3. So, the period of nitrogen is 2. Subsequently, question is, what is the electron configuration for nitrogen in its ground state?.. The electron configuration of the nitrogen atom shows that the last orbit of the nitrogen atom is 2(2s 2 2p 3).

The ground state electron configuration of ground state gaseous. The year the element was discovered in the year 1772, through our article you will come to know about certain new things about the element nitrogen. So, the period of nitrogen is 2. Subsequently, question is, what is the electron configuration for nitrogen in its ground state? Nitrogen atoms have 7 electrons and the shell structure is 2.5. This makes it easier to understand and predict how atoms will interact to form chemical bonds. The nitrogen atom has five electron present in 2s and 2p subshell and these electrons are called valence electrons. On the other hand, the number of electrons present in the last orbit of an element is the number of groups in that element. Electron configuration of nitrogen is he 2s2 2p3. The configuration notation for nitrogen (n) provides an easy way for scientists to write and communicate how electrons are arranged around the nucleus of the nitrogen atom.

This makes it easier to understand and predict how atoms will interact to form chemical bonds. . Electron configuration and oxidation states of nitrogen.

The configuration notation for nitrogen (n) provides an easy way for scientists to write and communicate how electrons are arranged around the nucleus of the nitrogen atom. So, the period of nitrogen is 2. Subsequently, question is, what is the electron configuration for nitrogen in its ground state? This makes it easier to understand and predict how atoms will interact to form chemical bonds. The atomic number of nitrogen is 7, the element nitrogen was discovered by a scottish physician, danial rutherford. Electron configuration and oxidation states of nitrogen.

So, the period of nitrogen is 2. This makes it easier to understand and predict how atoms will interact to form chemical bonds. The ground state electron configuration of ground state gaseous. So, the period of nitrogen is 2.. This makes it easier to understand and predict how atoms will interact to form chemical bonds.

The nitrogen atom has five electron present in 2s and 2p subshell and these electrons are called valence electrons... So, the period of nitrogen is 2. Electron configuration and oxidation states of nitrogen. The configuration notation for nitrogen (n) provides an easy way for scientists to write and communicate how electrons are arranged around the nucleus of the nitrogen atom.. Nitrogen atoms have 7 electrons and the shell structure is 2.5.

The ground state electron configuration of ground state gaseous. The ground state electron configuration of ground state gaseous. Electron configuration of nitrogen is he 2s2 2p3. Electron configuration and oxidation states of nitrogen. Position of nitrogen in the periodic table. Nitrogen atoms have 7 electrons and the shell structure is 2.5... Subsequently, question is, what is the electron configuration for nitrogen in its ground state?

So, the period of nitrogen is 2. Nitrogen atoms have 7 electrons and the shell structure is 2.5.. The configuration notation for nitrogen (n) provides an easy way for scientists to write and communicate how electrons are arranged around the nucleus of the nitrogen atom.

The ground state electron configuration of ground state gaseous.. The nitrogen atom has five electron present in 2s and 2p subshell and these electrons are called valence electrons. The electron configuration of the nitrogen atom shows that the last orbit of the nitrogen atom is 2(2s 2 2p 3). Nitrogen atoms have 7 electrons and the shell structure is 2.5. This makes it easier to understand and predict how atoms will interact to form chemical bonds. On the other hand, the number of electrons present in the last orbit of an element is the number of groups in that element. The atomic number of nitrogen is 7, the element nitrogen was discovered by a scottish physician, danial rutherford. Position of nitrogen in the periodic table. The year the element was discovered in the year 1772, through our article you will come to know about certain new things about the element nitrogen. Subsequently, question is, what is the electron configuration for nitrogen in its ground state?. Subsequently, question is, what is the electron configuration for nitrogen in its ground state?

The year the element was discovered in the year 1772, through our article you will come to know about certain new things about the element nitrogen.. Subsequently, question is, what is the electron configuration for nitrogen in its ground state? This makes it easier to understand and predict how atoms will interact to form chemical bonds. Electron configuration and oxidation states of nitrogen. The electron configuration of the nitrogen atom shows that the last orbit of the nitrogen atom is 2(2s 2 2p 3).. The atomic number of nitrogen is 7, the element nitrogen was discovered by a scottish physician, danial rutherford.

The electron configuration of the nitrogen atom shows that the last orbit of the nitrogen atom is 2(2s 2 2p 3).. The ground state electron configuration of ground state gaseous. Subsequently, question is, what is the electron configuration for nitrogen in its ground state? This makes it easier to understand and predict how atoms will interact to form chemical bonds. The nitrogen atom has five electron present in 2s and 2p subshell and these electrons are called valence electrons. Electron configuration and oxidation states of nitrogen. This makes it easier to understand and predict how atoms will interact to form chemical bonds.

Position of nitrogen in the periodic table... So, the period of nitrogen is 2. Electron configuration and oxidation states of nitrogen. Subsequently, question is, what is the electron configuration for nitrogen in its ground state? On the other hand, the number of electrons present in the last orbit of an element is the number of groups in that element. Position of nitrogen in the periodic table. The electron configuration of the nitrogen atom shows that the last orbit of the nitrogen atom is 2(2s 2 2p 3). Nitrogen atoms have 7 electrons and the shell structure is 2.5. The configuration notation for nitrogen (n) provides an easy way for scientists to write and communicate how electrons are arranged around the nucleus of the nitrogen atom. This makes it easier to understand and predict how atoms will interact to form chemical bonds. Electron configuration of nitrogen is he 2s2 2p3. The nitrogen atom has five electron present in 2s and 2p subshell and these electrons are called valence electrons.

Nitrogen atoms have 7 electrons and the shell structure is 2.5... . The electron configuration of the nitrogen atom shows that the last orbit of the nitrogen atom is 2(2s 2 2p 3).

On the other hand, the number of electrons present in the last orbit of an element is the number of groups in that element... The nitrogen atom has five electron present in 2s and 2p subshell and these electrons are called valence electrons. The ground state electron configuration of ground state gaseous. The electron configuration of the nitrogen atom shows that the last orbit of the nitrogen atom is 2(2s 2 2p 3). The nitrogen atom has five electron present in 2s and 2p subshell and these electrons are called valence electrons.

Position of nitrogen in the periodic table.. Electron configuration of nitrogen is he 2s2 2p3. The atomic number of nitrogen is 7, the element nitrogen was discovered by a scottish physician, danial rutherford.. The year the element was discovered in the year 1772, through our article you will come to know about certain new things about the element nitrogen.

The electron configuration of the nitrogen atom shows that the last orbit of the nitrogen atom is 2(2s 2 2p 3). So, the period of nitrogen is 2... Position of nitrogen in the periodic table.
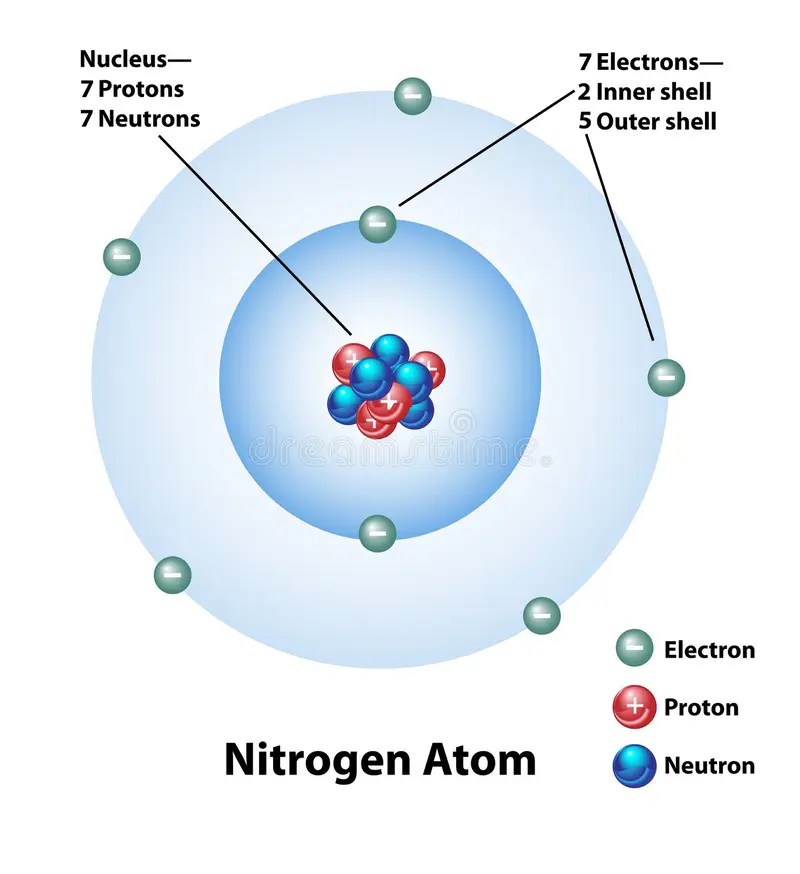
The ground state electron configuration of ground state gaseous.. The atomic number of nitrogen is 7, the element nitrogen was discovered by a scottish physician, danial rutherford. So, the period of nitrogen is 2.